Storage of vaccinations and other refrigerated or frozen medications outside recommended temperatures can cause irreversible loss of potency and ultimately treatment failure. With vaccines, the risks can be even higher because the patient and provider will both think they are protected when, in fact, the patient failed to develop an immune response from the vaccine. When this is discovered (which happens on rare occasions) the patient must be revaccinated. Patients can lose trust in the healthcare system and in vaccines when this occurs.
Unfortunately, the cold chain is still broken regularly; in fact, a VFC survey in 2012 found that 76% of provider offices surveyed over a two-week period had exposed vaccines to temperatures outside manufacturer recommendations.
Sometimes a picture really does say a thousand words; just look at the pictures on pages 16 and 20 of that survey and note the ice formation on the vaccine box, as well as around the freezer of a dorm unit refrigerator where vaccines were being stored.
In addition, take a look at this picture to see just how one pharmacy received vaccines from provider offices being transported there during an emergency:
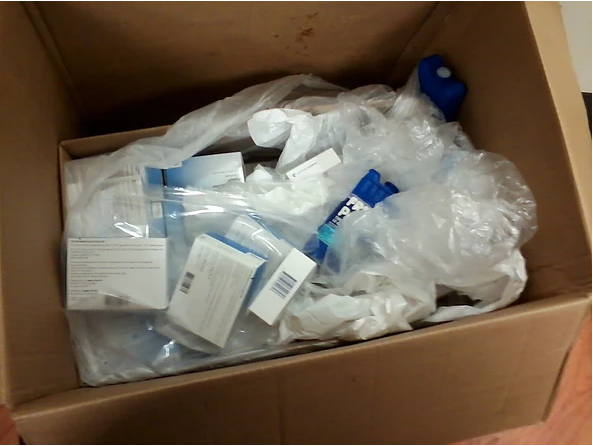
Why does this matter? After all, the vaccines look exactly the same after they've been exposed, and we can draw them up in a syringe and give them just as easily.
For this article we're going to look at a sampling of things in your refrigerator and dig into some of the mechanisms behind their demise to better understand why the cold chain is so important.
Inactivated vaccines
Aluminum adjuvants
Aluminum has a 70+ year history of safety as an adjuvant and is added to most inactivated vaccines (especially when only a component of the antigen is used) to boost the immune response. It has been shown to:
-
Increase the immune cells' affinity for the site of injection
-
Increase the uptake of antigen by the immune cells
-
Activate antibody response to the antigen
In other words, without the aluminum the vaccine would fail to provide enough of an immune response to produce immunity.
When exposed to freezing temperatures, the aluminum irreversibly conglomerates and thus cannot do its job. While there are multiple aluminum salts that can be used and significant research going into improving the thermal stability of aluminum-containing vaccines, the fact of the matter is that today's aluminum-containing vaccines are sensitive to freezing temperatures.
Infectious agent components
Inactivated vaccines are typically composed of just components of the infectious agent, not the entire agent (as in live vaccines). Those components can be surface proteins, like hemagglutinin in the case of the influenza vaccine. Those proteins degrade much more quickly when exposed to elevated temperatures; for example, one study showed that the hemagglutinin degrades 6-12 times faster at 25C than it does at 5C.
Other studies have shown that exposure to freezing temperatures causes unfolding of the hemagglutinin protein.
In either case, the immune system will no longer be able to recognize the protein and thus will not mount an immune response to it.
Live Vaccines
Higher than recommended temperatures
Live vaccines are freeze-dried (lyophilized) and stored frozen to maintain their stability. They are particularly sensitive to higher than recommended temperatures.
There is some data on the stability of both the polio as well as the measles vaccine when exposed to elevated temperatures. The measles vaccine is especially unstable when exposed to elevated temperatures and undergoes rapid degradation; in the case of the polio vaccine, elevated temperatures cause degradation of the infectious components (the capsid protein and viral RNA). In both cases, the ultimate result could be a vaccine that is ineffective.
Insulin
Freezing
While the data on the stability of insulin exposed to freeze-thaw cycles suggests most insulins can withstand some exposure to freezing temperatures, insulin is prone to developing particulates and undergoing structural changes that can lead to reduced efficacy.
Higher than recommended temperatures (above 46F)
While all insulins on the market have stability data to support their being out at room temperature for a specified period of time, there is less data to support insulins exposed to temperatures above 46F being placed back in the refrigerator and still used through the expiration date.
When exposed to higher temperatures, insulin undergoes rapid degradation through hydrolysis or the formation of aggregate and higher molecular-weight products and quickly loses potency. Insulins commonly form dimers but at higher temperatures can even form oligomers and polymers. Protamine-containing insulins (i.e. NPH and 70/30) will form protamine-insulin byproducts. Just as in the previous examples, the end result is insulin that is ineffective.
Biologics
TNF-alpha inhibitors
One study exposed three TNF-alpha inhibitors (etanercept [Enbrel], adalimumab [Humira] and certolizumab pegol [Cimzia]) to freezing either through multiple freeze-thaw cycles or through constant exposure to freezing for 96 hours, followed by thawing. Half of the stressed products contained an increased number of particulates when compared to controls.
While it is true that the increased number of particulates was only seen in some of the methods used to analyze the syringes, it is certainly better safe than sorry for these.
Other Blogs you may be Interested In...
- How Storage Temperature Impacts Vaccine Degradation
- Reducing Risk Related to Improper Vaccine Storage
- A Look into the 2019-2020 Flu Season
- The Ultimate Guide to CDC Vaccine Storage and Temperature Monitoring
- Routine Maintenance & Troubleshooting for Vaccine Storage Units
Summary
The cold chain protects the most valuable inventory in your pharmacy and there is significant data to support the susceptibility of a wide range of medications exposed to out of range temperatures when the cold chain is broken. In addition, dispensing medications that have been stored outside recommended temperatures and without manufacturer data to support their stability is not only unsafe but can pose a liability risk to the pharmacy as well.
For more help on protecting the refrigerated and frozen medications in your pharmacy, check out my collection of helpful links.
For helpful tips on handling a temperature excursion, check out this article I wrote on the subject. In addition, if you are interested in learning more about this topic I highly recommend the review article by Kumru, et al. linked here and referenced below.
This post originally appeared on www.pharmcompliance.com.
References:
Kumru O, Joshi S, Smith D, Middaugh CR, Prusik T, Volkin D. Vaccine instability in the cold chain: Mechanisms, analysis and formulation strategies. Biologicals42 (2014) 237e259.
Tanya Clapp, Paul Siebert, Dexiang Chen, and LaToya Jones Braun.Vaccines with Aluminum-Containing Adjuvants: Optimizing Vaccine Efficacy and Thermal Stability. J Pharm Sci. 2011 Feb; 100(2): 388–401.
Centers for Disease Control. Adjuvants to help vaccines work better. https://www.cdc.gov/vaccinesafety/concerns/adjuvants.html. Accessed July 31, 2019.
Chandler C, Gryniewicz C, Pringle T, Cunningham F. Insulin temperature and stability under simulated transit conditions. American Journal of Health-System Pharmacy, Volume 65, Issue 10, 15 May 2008, Pages 953–963. https://academic.oup.com/ajhp/article/65/10/953/5128317.
Vlieland ND, Nejadnik MR, Gardarsdottir H, Romeijn S, Sediq AS, Bouvy ML, Egberts ACG, van den Bemt BJF, Jiskoot W. The Impact of Inadequate Temperature Storage Conditions on Aggregate and Particle Formation in Drugs Containing Tumor Necrosis Factor-Alpha Inhibitors. Pharm Res. 2018 Feb 5;35(2):42. doi: 10.1007/s11095-017-2341-x. https://www.ncbi.nlm.nih.gov/pubmed/29404710
Vimalavathini R, Gitanjali B. Effect of temperature on the potency & pharmacological action of insulin. Indian J Med Res. 2009 Aug;130(2):166-9. https://pdfs.semanticscholar.org/6ca3/2a2e8b75e8818e048334be15335d5f625c60.pdf
Gregory R, Edwards S, Yateman NA. Demonstration of insulin transformation products in insulin vials by high-performance liquid chromatography. Diabetes Care. 1991 Jan;14(1):42-8. https://www.ncbi.nlm.nih.gov/pubmed/1991434
Brange, J, Havelund S, Hougaard, P. Chemical Stability of Insulin. 2. Formation of Higher Molecular Weight Transformation Products During Storage of Pharmaceutical Preparations. Pharm Res (1992) 9: 727. https://doi.org/10.1023/A:1015887001987.
CDC Vaccine Storage and Handling Toolkit. Centers for Disease Control. Accessed online July 16, 2019 at https://www.cdc.gov/vaccines/hcp/admin/storage/toolkit/storage-handling-toolkit.pdf.




