In this blog post, Alex Evans, PharmD, CGP, from Pharmaceutical Compliance gives us an important look into temperature monitoring for pharmaceutical grade refrigerators and freezers.
The temperature monitoring device (TMD) being used to ensure the viability of the refrigerated and frozen vaccines and medications stored in the pharmacy is an often-overlooked area.
Unfortunately, inaccuracy can lead to the administration or dispensing of medications that have been stored outside of recommended temperatures.
Why is this a problem?
Vaccines, and any medication, stored outside manufacturer-recommended temperatures can quickly lose potency and thus efficacy. Administering a vaccine that has been stored improperly can be worse than not administering a vaccine at all because the patient will believe they are protected when in fact, they are not.
In addition, administration and storage errors can breed public distrust in vaccines and healthcare as a whole. It may also expose the pharmacist and the pharmacy to potential liability.
That being said, here are some important considerations when choosing a thermometer for your pharmacy:
1. TMD type: According to the CDC Vaccine and Storage Handling Toolkit, a Digital Data Logger (DDL) is the preferred type of TMD. The primary advantage of a DDL is that it can record temperatures automatically in intervals; the CDC recommends that a DDL record at least every 30 minutes. These recordings are typically downloadable onto an Excel spreadsheet. There are now many types of thermometers that can send data and/or alerts to a smartphone as well.
In the past, TMDs that would only record a min-max temperature over a 24 hour period, but this could lead to a lot of waste in the case of an excursion because the pharmacist would be unable to tell how long the vaccine or medication was out of the recommended range.
I remember an incident where the pharmacy had an excursion and was recording temperatures twice daily, including a min-max (the standard of practice at the time). Unfortunately, that means the readings were about 12 hours apart and we had a medication that the manufacturer had stability data for going out 8 hours, but not 12, so we had to throw it away. It was a $3,000 medication! Moral of the story - invest in a quality temperature monitoring device.
Types that are not recommended by the CDC include:
-
Food thermometers
-
Thermometers that measure air temperature only
-
Chart recorders
-
Mercury or alcohol-filled thermometers
2. Certificate of Traceability: Just like a pedigree in fine art tracing ownership of the work back to the artist (or even a pedigree in DCSCA compliance tracing a drug back to the manufacturer), a TMD calibration must be traced all the way back to global uniform standards.
In the US, the National Institute of Standards and Technology ensures uniform measurement standards; globally, the BIPM (Bureau International des Poids et Mesures), consisting of member-states, ensures accuracy of measurement.
3. Certificate of Calibration: TMDs, like any other instrument, must be calibrated to ensure accuracy. Just like in medicine and many other fields if it isn't documented, it wasn't done. Very important to note - these certificates expire; when they do, the thermometer must be replaced.
While the CDC does note that you can send it off for calibration testing again and replace it if it fails (note: calibration does not necessarily mean adjusting the TMD - it only means measuring its accuracy), temperature monitoring devices are cheap enough this is not really practical or worth it. In other words, temperature monitoring devices do expire and must be replaced!
In summary, a Certificate of Traceability ensures the instrument used to calibrate your TMD is accurate to global standards, while the Certificate of Calibration ensures the accuracy of your TMD.
4. Other features:
-
Buffered probe (often filled with glycol, and intended to mimic conditions inside the vial of the vaccine)
-
Alarm for out-of-range temperatures (again, many offer alarms connected to a smartphone)
-
Current as well as min-max display
-
Recommended uncertainty of +/- 0.5 degrees Celsius (+/-1 Fahrenheit)
Although not a CDC recommendation, I personally recommend having two TMDs per refrigerator (CDC recommends just one for the facility as a backup). It is a minimal additional cost (especially compared to the cost of all the medications and vaccines being stored in the refrigerator).
I have seen devices have problems occasionally - batteries die (best practice is to plug it in and not use batteries, by the way), a button gets hit and it stops reading, it turns off, etc. Just see the below pictures I took in one of my pharmacies where that actually happened (both pictures were taken at the same time).
Other Blogs you may be Interested In...
- How Storage Temperature Impacts Vaccine Degradation
- Mechanisms of Vaccine and Refrigerated Medication Degradation
- Reducing Risk Related to Improper Vaccine Storage
- Where to Properly Place a Temperature Probe in Your Vaccine Refrigerator
- The Ultimate Guide to CDC Vaccine Storage and Temperature Monitoring
Upon further investigation, I confirmed it did not reach 32 degrees; the TMD was listing a previous low that was prior to it being placed in this refrigerator. Having my backup gave me peace of mind while I investigated the one that went awry.

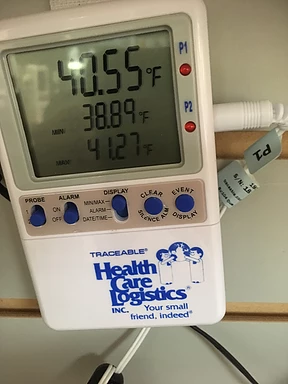
Of note, here I am referring to two thermometers that are recording the temperature at the same time, not one that it is in the refrigerator and one sitting in a box somewhere.
Ensuring that you are up to date with the latest guidelines on vaccine storage and handling can be a daunting task. One of the best places to start, in addition to the CDC Vaccine Storage and Handling Toolkit, is www.immunize.org, which has numerous free resources related to vaccine storage, administration, education, and more.
Helmer Scientific's Ultimate Guide to Vaccine Storage highlights additional CDC storage and temperature monitoring recommendations. You can download the guide at the link below.
This post originally appeared on www.pharmcompliance.com.




