This week, we are continuing our Medication Safety Officer blog series on managing refrigerated medications. Each week, Network Medication Safety Officer, Jessalynn Henney, PharmD, will be answering questions related to the safety and security of storing and handling refrigerated medications. If you missed any of the previous posts, you can find them at the following links:
- Week one: What are some of the factors influencing dispensing errors?
- Week two: How does using automated dispensing cabinets (ADCs) benefit the health system?
- Week three: Could you please explain the difference between LASA medications and look alike packaging, and provide a few examples of potential medication errors that could be seen in practice?
This week, we are talking about what refrigerated medications should be securely stored within locked and lidded compartments within the refrigerator due to safety, cost, and clinical value.
What refrigerated medications would have the biggest impact on medication management if stored in discrete segregated bins?
I would first focus on medications with safety concerns, such as high alert or LASA medications. Then those with higher cost and higher value (i.e. clinical importance). While a small percentage of medications require refrigeration (~2% is what I have seen in practice), a majority of the medication requiring this temperature storage have a higher concern with safety, cost, or clinical value.
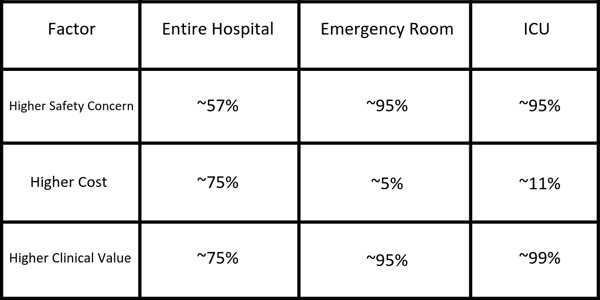
While many facilities have discrete, locked lidded bins for medications stored at room temperature, this strategy has not been implemented for medications requiring refrigerated storage. I recommend reviewing the types of medications stored at refrigerated temperatures and ensuring the same safety strategies are put into place for these medications. Refrigerated medications having any of these three factors (safety, cost, and clinical value) should be given extra attention when it comes to storage and organization within the refrigerator.
When doing monthly rounds of the medication rooms, ensure specific actions to review the refrigerator are completed. Examples of these tasks could include; confirming all bins are placed in an orderly fashion and appropriately labeled, double checking LASA and look like packaging medications are not stored adjacent, separating high alert medications, inspecting bin size for appropriateness, and utilizing lidded bins.
How can facilities ensure these specific drugs are stored safely and in a way that reduces errors?
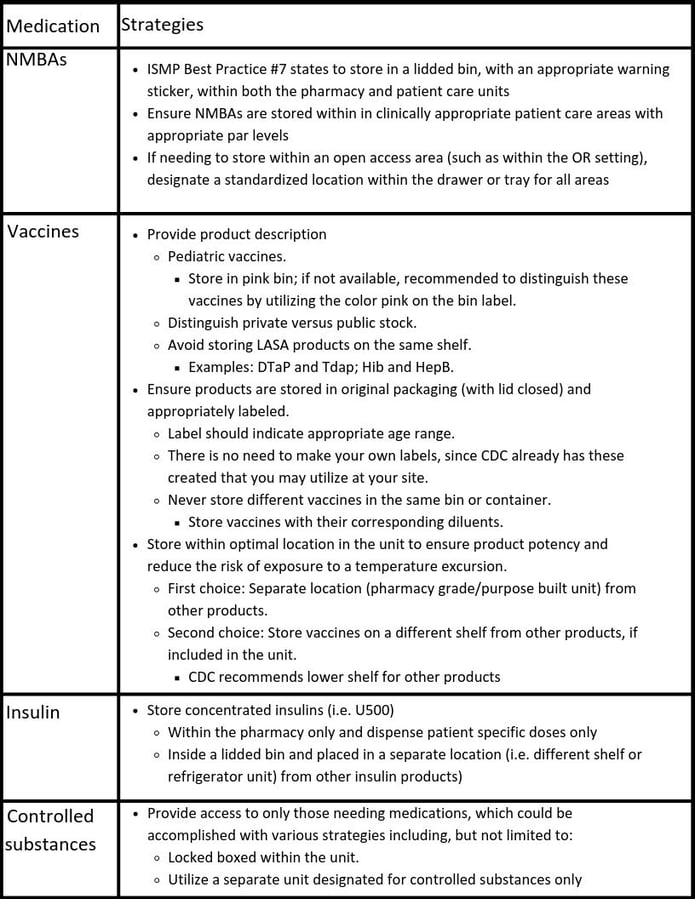
Focusing on medications with higher safety concerns, such as High Alert or LASA medications, I find ISMP and CDC provide excellent references to help guide safe practices and assist facilities to develop simple strategies, especially focusing on safe storage of neuromuscular blockers (NMBAs), vaccines, insulin, and controlled substances.
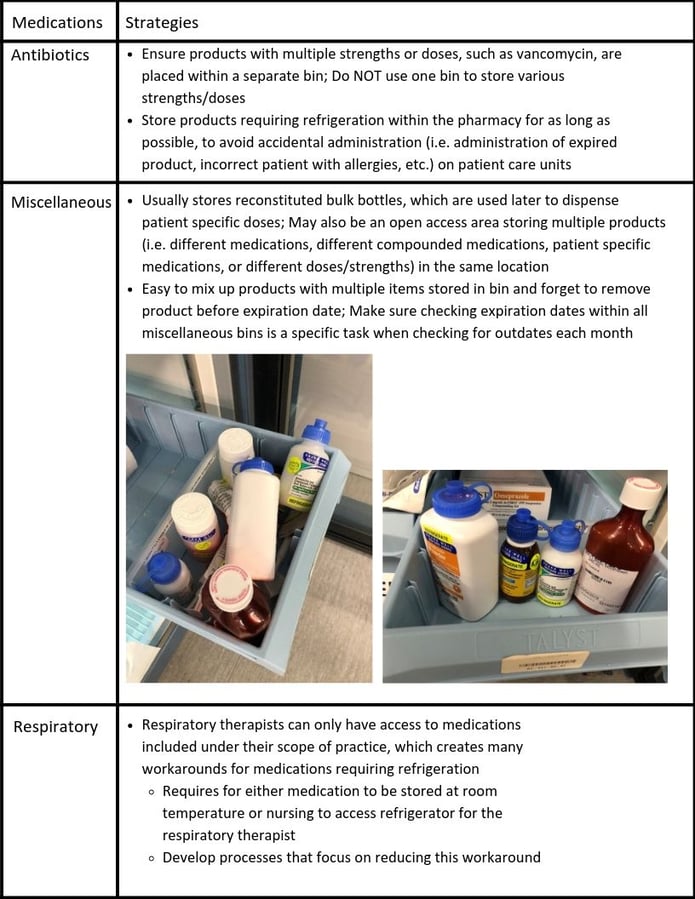
Outside of these medications, additional medications or storage areas to focus on where I have seen a multitude of errors include; antibiotics, miscellaneous bins, and respiratory medications.
Other Blogs You Might Be Interested In...
- A Medication Safety Officer’s Perspective on Safely Managing Refrigerated Medications: Week One
- A Medication Safety Officer’s Perspective on Safely Managing Refrigerated Medications: Week Two
- A Medication Safety Officer’s Perspective on Safely Managing Refrigerated Medications: Week Three
- USP <800> Refrigerator Frequently Asked Questions Answered