In 2012, the Department of Health and Human Services (DHHS), Office of Inspector General, conducted a study called, Vaccines for Children Program: Vulnerabilities in Vaccine Management. The study was conducted to gauge how well VFC providers were meeting certain requirements for vaccine storage and handling, such as storing vaccines within required temperature ranges and monitoring expiration dates, to ensure that these vaccines provide children with maximum protection against preventable diseases. These requirements are also intended to decrease VFC program fraud, waste, and abuse.
DHHS used Centers for Disease Control and Prevention (CDC) data to select 45 VFC providers from the 5 grantees with the highest volume of vaccines ordered in 2010. The study consisted of site visits, interviews, observation, and independent temperature monitoring. None of the sites met all the VFC requirements. Over 75% of the 45 providers had vaccines stored at out of range temperatures for at least 5 cumulative hours, and thirteen providers had expired vaccine stored with nonexpired vaccines. All these errors present risk.
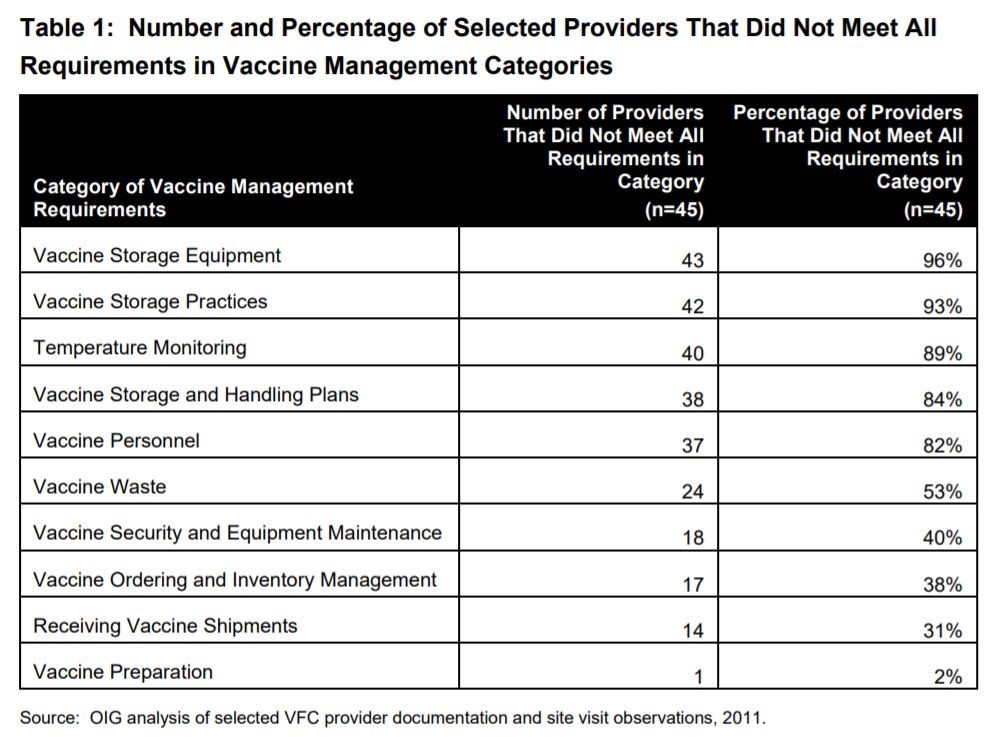
Out of this study, the DHHS made very clear recommendations to the CDC to move the VFC program forward. Some of the vaccine storage and handling recommendations included:
- Ensure That VFC Vaccines Are Stored According to Requirements
- Ensure That Expired VFC Vaccines Are Identified and Separated From Nonexpired Vaccines
- Ensure That Grantees Better Manage Providers’ Vaccine Inventories
- Ensure That Grantees Meet Oversight Requirements
Vaccination is one of the most successful public health tools in preventing and controlling disease. CDC agreed that all four recommendations were essential in improving the VFC program. Out of these recommendations the CDC Vaccine Storage and Handling Toolkit was born. The toolkit is now widely available and found in most healthcare settings where vaccines are administered. However, enhancing the vaccine program is an ongoing effort and takes contribution from every practice around the country. New research, technology and equipment will drive the future of vaccine storage and handling, therefore, improving vaccination rates and overall public health. To read the full report from 2012, follow the link below.




