Ensuring vaccines remain within recommended temperatures is critical for safe and effective immunizations. Choosing the right vaccine refrigerator and freezer for these life-saving products can help avoid temperature excursions.
Continuous monitoring systems and data loggers identify temperature excursions. However, identifying vaccines stored outside of recommended ranges in refrigerators and freezers with poor temperature uniformity can be challenging if only one location within the cabinet is being measured.
The U.S. Centers for Disease Control (CDC) provides recommendations and guidance for clinicians related to vaccine storage in the CDC Vaccine Storage and Handling Toolkit. This toolkit recommends the use of purpose-built or pharmaceutical-grade units and to avoid cold storage units not designed for clinical use, such as dormitory-style units. It also provides caution for units that could have wide variation of temperatures across storage locations within a cabinet.
Because the CDC understands hot and cold spots within these types of units may not be obvious, recommendations include avoiding storage areas on door shelves or floors, as well as loading certain storage spaces with water bottles to help maintain temperature.
These extra steps and safeguards are not needed for medical-grade equipment, which is designed to maintain consistent temperatures across all potential storage areas within a cabinet.
Most facilities are only monitoring single locations within the cabinet using data loggers or continuous monitoring systems. While a single-probe location monitoring system could indicate a vaccine refrigerator or freezer is within range, there may still be some storage locations outside of safe ranges for vaccines that aren't easily detectable. If this is the case, vaccines may be exposed to temperatures that are too high, too low, or even freezing for refrigerated products.
Temperature mapping studies are designed to help characterize the uniformity and consistency of temperatures in vaccine refrigerators and freezers. It is not always practical for a facility to perform their own temperature validation studies that map usable storage space throughout a refrigerator or freezer cabinet; however, these studies are routinely performed by medical-grade cold storage manufacturers.
Helmer Scientific vaccine refrigerators and freezers have been designed and tested to ensure uniformity is +/- 1°C. Helmer units will maintain consistent temperatures across all storage locations, regardless of location on a shelf or a drawer, and the units do not require special guidance related to usable storage locations or the use of water bottles to help ensure products stay within recommended range.
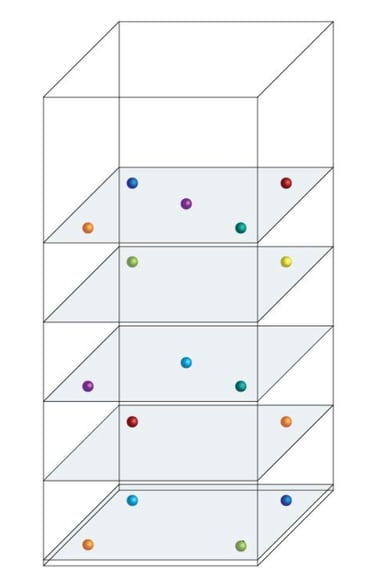
Example probe locations in an upright refrigerator cabinet for temperature uniformity studies
The NSF/ANSI 456 Vaccine Storage Standard, published in 2021, builds upon the recommendations found in the CDC Vaccine Storage and Handling Toolkit related to use of purpose-built and pharmaceutical grade cold storage. The standard includes temperature performance requirements that require manufactures to verify uniformity during a range of product load conditions and door-open scenarios that simulate clinical use.
To pass this standard, all locations must maintain required temperature during normal use, which includes a series of frequent door openings. In addition, all locations within the cabinet must recover quickly to required temperature ranges after extended door openings that simulate time needed for inventory management or cleaning.
Helmer Scientific's GX Solutions refrigerators and freezers have been tested and certified to this new standard through Intertek Laboratories. As part of this certification process, Helmer units were tested using probes across 15 different locations within the cabinet.
OTHER BLOGS YOU MIGHT BE INTERESTED IN...
- Emerging Standards for Vaccine Storage in 2021
- Medical-grade vaccine storage can help prevent vaccine and financial loss
- Explaining the NSF / ANSI 456 Vaccine Storage Standard
- Helmer's GX Solutions Certified to NSF/ANSI Vaccine Storage Standard
Selecting vaccine storage units tested and certified to NSF/ANSI 456 Vaccine Storage Standard, such as Helmer's GX Solutions refrigerators and freezers, can help provide confidence in basic temperature uniformity characteristics.
Our white paper includes more information related to the NSF/ANSI 456 Vaccine Storage Standard, as well as examples of temperature mapping data generated through testing.





