
In 2010, approximately 82 million vaccine doses were administered to an estimated 40 million children in the United States through the Vaccines for Children (VFC) program at a cost of $3.6 billion. Vaccines must be stored and handled appropriately throughout the cold chain (from manufacturing to administration) to remain effective. Excessive exposure to heat or freezing can cause vaccines to lose potency, meaning the patients who receive the compromised vaccines will not be protected. Studies conducted over the years highlight improper vaccine storage as a serious problem in the United States.
- A survey conducted in 2008 by the Association of Immunization Managers (AIM) demonstrated considerable variability among city and state immunization projects in the United States.
- A field survey conducted by the California Department of Public Health found that a hospital in Santa Ana, Calif, had stored various vaccines at lower than freezing temperatures for 7 months in 2009. As a result, 1641 newborns were given potentially defective hepatitis B vaccine.
- In 2010, another investigation found a significant 76% correlation between the 25% of vaccine refrigerators in Houston's community health centers that experienced prolonged freezing temperatures and that region's rate of pertussis.
- In 2014, the Hartford (Connecticut) HealthCare Medical Group reported that the effectiveness of 5003 vaccinations given to 3833 patients since January 2013 may have been compromised because of poor temperature control. Patients, including those vaccinated against pneumonia or pertussis, were to be revaccinated.
These earlier findings paved the way for conducting an in-field, observational study with the hopes of helping VFC providers better manage vaccines to avoid waste and inadvertent administration of ineffective vaccines to children. The primary objective of the study was to determine whether the help of visual freeze indicators on vaccine boxes would assist health care providers in identifying vaccines that have been exposed to out of range temperatures.
The study, Visual Indicators on Vaccine Boxes as Early Warning Tools to Identify Potential Freeze Damage, was conducted for 24 weeks at 27 VFC provider sites across Connecticut. Researchers measured:
- Freeze events indicated by the visual freezer indicators
- Freeze events identified by temperature monitor readings
- Various temperature points inside of the cabinets
- Type, number of doses, and value of vaccine with triggered visual freeze indicators
- Total inventory value of vaccine that triggered a freeze event
- Site and vaccine refrigerator characteristics (monitoring device, type of refrigerator, location in the refrigerator).
All 27 sites experienced a “freeze” temperature excursion and some sites as many as 6. The study verified that, in general, VFC providers still need to invest in proper cold storage equipment to ensure safe and effective vaccine management.
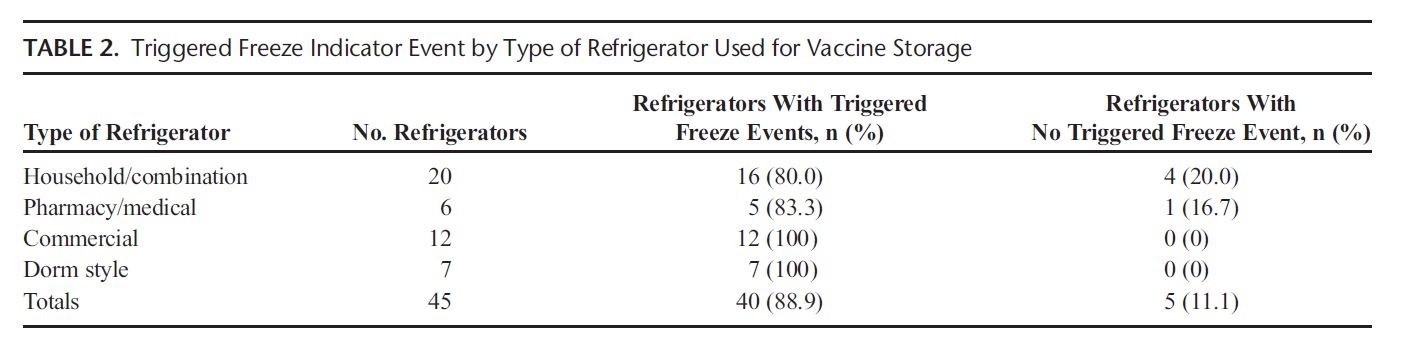
Freeze events are much more common than previously reported. Providers and patients are faced with important implications when vaccines are not stored correctly. Thanks to soaring vaccine prices, many providers report extensive financial losses due to improperly stored vaccine. There is also great expense associated with revaccination. Lastly, patient access to vaccines may be compromised due to vaccine wastage, which places children at risk of contracting preventable diseases. The right processes, equipment, and training can help mitigate these risks. The CDC toolkit is a great place to begin. To access the toolkit, follow the link below.
Reference: https://pdfs.semanticscholar.org/29b3/a9b090683b8b4e3983e2443e4400c053432e.pdf
Angoff, Ronald, et al. Visual Indicators on Vaccine Boxes as Early Warning Tools to Identify Potential Freeze Damage. pdfs.semanticscholar.org/29b3/a9b090683b8b4e3983e2443e4400c053432e.pdf.




