
At the 2018 ASHP Summer Meeting, one presentation discussed the 14 latest ISMP best practices to combat medication errors. More than 2,000 “events” have been submitted to ISMP’s national medication errors and vaccine errors reporting program from March 2017 through February 2018.
Drug Shortage Medication Errors
Drug shortages are a contributing factor to medication errors. Nearly one-fourth of people surveyed by ISMP in 2017 said they knew of an error directly related to a drug shortage. Many of the errors are a result of using unfamiliar or different devices. It is recommended to keep staff aware and up-to-date of shortages and changes.
Vaccine Medication Errors
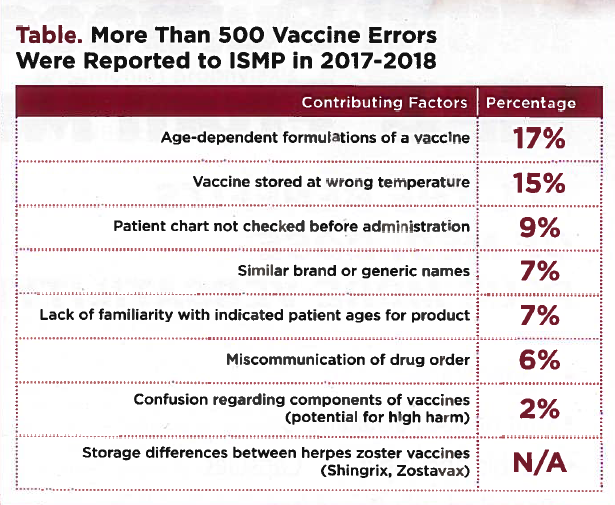
Improper labeling is a frequent offender resulting in vaccine errors. Common contribution factors to errors involving vaccines include using the wrong age-dependent formulation, storing vaccines at the wrong temperatures, not checking patient charts before administration, using a similar brand or generic name and miscommunicating orders, confusion regarding the components of two-component vaccines (patients only receiving the diluent), and specific issues around storage of the herpes zoster vaccine Shingrix. Many of these errors are related to poor workflow.
Labeling & Packaging Medication Errors
Labeling is another major issue when it comes to compounded syringes. These products may not adhere to the same standard as FDA-approved products. In addition, drugs made outside of the United States have different labels. The various label types can lead to confusion and error. The International Medication Safety Network is looking at ways to standardize medication labeling and packaging to reduce error.
New technology is set to hit the market to help enhance the safety and efficiency of medication administration workflows. The goal of the new technology is to reduce medication errors and assist medication safety, pharmacists and nursing in following ISMP best practices.




