Proper vaccination is the CDC’s primary recommendation to protect against seasonal flu. It is estimated that between 157 and 168 million doses of injectable vaccine will be administered during this year’s flu season alone. However, these life-saving vaccinations are only effective when stored properly under tight temperature controls. The CDC has developed the Vaccine Storage and Handling Toolkit to provide guidelines and best practices to safeguard vaccines and protect patients. As a continuation of this important work to ensure quality in vaccination programs, a NSF committee was formed in 2015 to create new standards specifically for vaccine storage equipment. This committee is comprised of representatives from NSF, NIST, CDC, clinicians, and vaccine storage manufacturers including Helmer Scientific.
Based on an article published in 2016 by NIST, one challenge for this committee was the lack of data related to typical use cases in clinics frequently administering vaccines. In particular, the frequency and length of door openings by the nurse or technician accessing vaccines was unknown. The frequency and length of door openings is extremely relevant when considering vaccine refrigerator and freezer performance. To close this data gap, NSF provided data loggers to over two dozen clinics across the United States to track door openings. The results of the initial evaluation were that doors were opened for an average of seven seconds, but could be accessed over 30 times in one hour! The evaluation also confirmed that prolonged door openings were common, likely due to vaccine restocking periods.
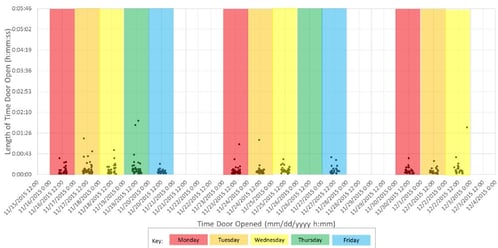
Using feedback from this evaluation is just one of the ways the NSF committee is developing protocols to test and create standards for vaccine storage.
Figure 1. Sample data from NSF Vaccine Refrigerator Door Opening Study https://www.nist.gov/news-events/news/2016/02/vaccine-fridge-field-study-opens-doors-new-standards
Future standards, which have not yet been released by the NSF, will help further clarify performance requirements of medical-grade refrigeration used for this important application. Performance attributes such as temperature recovery and uniformity can be critical when assessing safe practices for vaccine storage. New guidelines and standards will ultimately help eliminate healthcare provider confusion and ensure higher quality patient care. More information about this NSF initiative can be found in NIST News.
Interested in learning more about Vaccine Storage guidelines? Download the Ultimate Guide to Vaccine Storage using the link below.




