
Hazardous drugs are defined as drugs which involve risks when handled in occupational settings. Workers exposed to hazardous drugs are to be informed of the hazardous chemicals in the drugs they are handling as well as the potential side effects of exposure. Effects include but are not limited to acute toxicity (any route of exposure), skin corrosion and irritation, detrimental eye damage and irritation, respiratory and skin issues, germ cell mutagenicity, and organ toxicity.
Drugs are classified as hazardous if studies indicate that exposure to them can cause cancer, developmental or reproductive toxicity, or harm to organs (cdc). USP Chapter <800> segments HDs into three categories the same way as they are segmented in the NIOSH list. The following infographic defines the characteristics of hazardous drugs according to NIOSH.
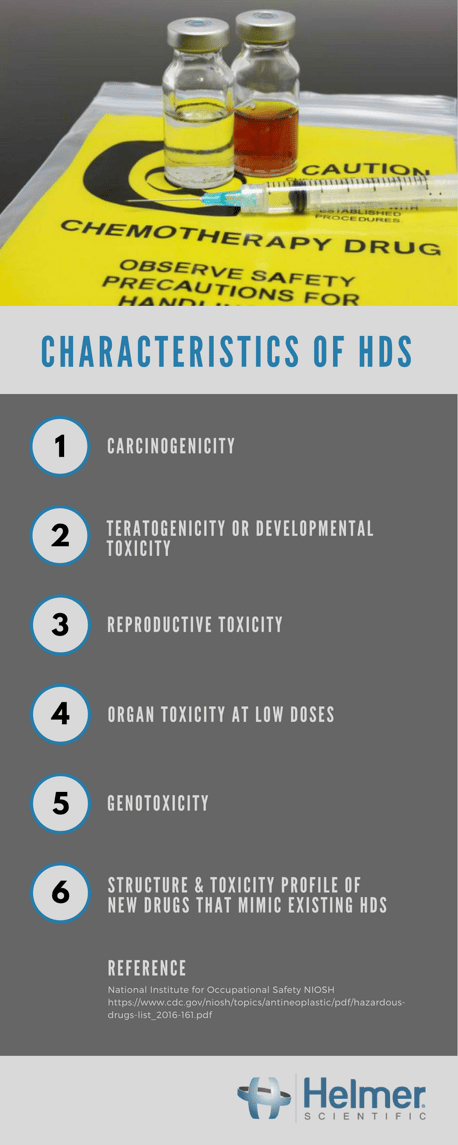
There have been guidance documents from the National Institute for Occupational Safety (NIOSH) available for decades on how to safely handle hazardous drugs (HDs). The American Society of Health-System Pharmacists (ASHP) and the Oncology Nursing Association (ONS) also have provided instructions on the safe handling of HD’s since the 1980’s, yet studies still show significant signs of contamination throughout the delivery of care.
With the implementation of USP <800>, these guidelines will no longer be optional. In December of 2019, facilities will have to comply with the set of standards set fourth by USP to reduce contamination, risk, and hazardous drug exposure. The goal of the chapter is to keep healthcare workers safe from the harmful effects of these chemicals. The chapter outlines administrative controls, environmental and engineering controls, work practice controls, containment supplemental engineering controls, personal protective equipment, and technology, all with the goal of reducing risk and keeping technicians, pharmacists, and nurses healthy.
Have more questions about safely handling hazardous drugs? Reach out blog post about the ASHP USP <800> answer book.




